Traditional Chinese Medicine Shows Success in Treating Heart Failure in Clinical Trial
New research shows qiliqiangxin, a traditional Chinese medicine, effectively reduces hospitalizations and cardiovascular deaths in heart failure patients with reduced ejection fraction (HFrEF). Credit: SciTechDaily.com
Qiliqiangxin, a traditional Chinese herb blend, effectively reduces heart failure hospitalizations and deaths, proving safe and beneficial in a large-scale clinical trial.
The traditional Chinese medicine qiliqiangxin reduces hospitalization for heart failure and cardiovascular death in patients with heart failure and a reduced ejection fraction (HFrEF), according to late-breaking research presented in a Hot Line session at ESC Congress 2023.[1]
Qiliqiangxin is a traditional Chinese medicine extract obtained from 11 types of herbs (Table 1).[2] In a pilot study, qiliqiangxin reduced N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels and improved heart failure symptoms in patients with HFrEF when added to established heart failure treatment.3 Preclinical studies have also indicated that qiliqiangxin has beneficial effects on attenuating myocardial fibrosis and cardiac remodeling.[4-7]
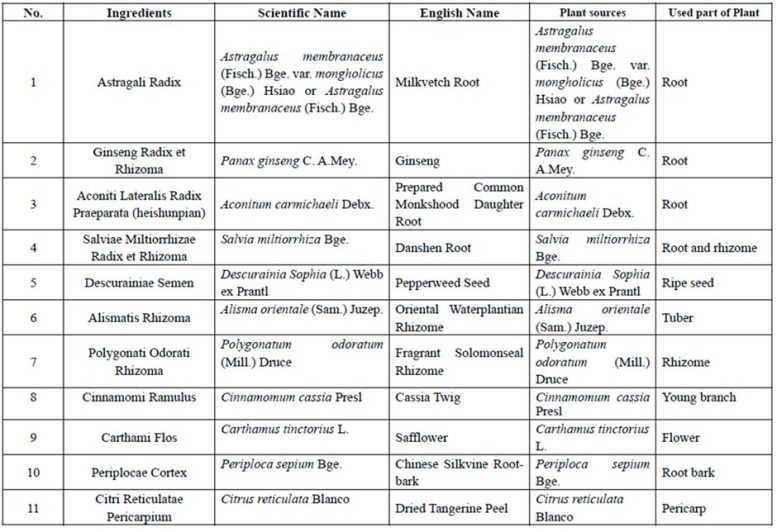
Table 1: Ingredients of qiliqiangxin. Credit: European Society of Cardiology
The QUEST Trial: A Comprehensive Evaluation
The QUEST trial evaluated the clinical efficacy and safety of qiliqiangxin on major heart failure outcomes in HFrEF patients. The trial was conducted at 133 hospitals in mainland China and Hong Kong SAR of China.
The trial enrolled adult HFrEF patients with a left ventricular ejection fraction of 40% or below and NT-proBNP of 450 pg/ml or higher who had been on a stable standardized baseline treatment regimen for at least two weeks prior to enrolment. Patients were randomized in a 1:1 fashion to receive qiliqiangxin (four capsules, three times daily) or placebo on top of standard medications for chronic heart failure. The primary endpoint was a composite of rehospitalization for worsening heart failure or cardiovascular death.
A total of 3,110 patients were included in the analysis, with 1,555 randomized to qiliqiangxin and 1,555 randomized to placebo. The average age was 62 years and 72.1% were men. At baseline, the mean left ventricular ejection fraction was 32%, and the median NT-proBNP was 1730.80 pg/ml.
Significant Reduction in Heart Failure Outcomes
During a median follow-up of 18.3 months, the primary endpoint occurred in 389 patients (25.02%) in the qiliqiangxin group and in 467 patients (30.03%) in the placebo group (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.68 to 0.90; p<0.001). This effect was related to both lower risks of rehospitalization for worsening heart failure (HR, 0.76; 95% CI, 0.64 to 0.90; p=0.002) and cardiovascular death (HR, 0.83; 95% CI, 0.68 to 0.996; p=0.045) in the qiliqiangxin group. The effect of qiliqiangxin on the primary outcome was generally consistent across prespecified subgroups including in the subgroups defined according to age and NT-proBNP level, and in patients with or without angiotensin receptor/neprilysin inhibitors (ARNIs).
Secondary Findings and Safety Analysis
In terms of secondary endpoints, the decrease in serum NT-proBNP between baseline and three-month follow-up was greater in the qiliqiangxin group (-444.00 [interquartile range -1401.00 to 85.00]) than in the placebo group (-363.00 [interquartile range -1280.00 to 183.00]) (p=0.047), which was consistent with the previous pilot study.3[]
Analysis of safety endpoints demonstrated no significant difference in all-cause mortality, which occurred in 221 patients (14.21%) in the qiliqiangxin group and 262 patients (16.85%) in the placebo group (HR, 0.84; 95% CI, 0.70 to 1.01; p=0.058). Qiliqiangxin capsules were well-tolerated, with no major differences between the two groups in adverse events including gastrointestinal symptoms, worsening renal function, and increased liver enzymes.
Expert Opinion and Conclusion
Principal investigator Professor Xinli Li of the First Affiliated Hospital of Nanjing Medical University, Nanjing, China said: “To our knowledge, this was the first randomized, double-blind controlled trial of a traditional Chinese medicine for the treatment of chronic heart failure. Our findings demonstrate meaningful clinical benefit with qiliqiangxin in patients with HFrEF, which support the use of qiliqiangxin as an adjunct therapy for treating heart failure.”
References
- QUEST was discussed during Hot Line 2 in room Amsterdam.
- Reference: “Study protocol for a randomized controlled trial: Qiliqiangxin in heart failUre: assESsment of reduction in morTality (QUEST)” by Wenming Yao, Iokfai Cheang, Shengen Liao, Yanli Zhou, Fang Zhou, Dongjie Xu, Zhenhua Jia, Liping Chang, Haifeng Zhang and Xinli Li, 5 February 2020, BMC Complementary Medicine and Therapies.
DOI: 10.1186/s12906-020-2821-0 - “A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study of the Effects of Qili Qiangxin Capsules in Patients With Chronic Heart Failure” by Xinli Li, Jian Zhang, Jun Huang, Aiqun Ma, Jiefu Yang, Weimin Li, Zonggui Wu, Chen Yao, Yuhui Zhang, Wenming Yao, Boli Zhang and Runlin Gao, 7 June 2013, Journal of the American College of Cardiology.
DOI: 10.1016/j.jacc.2013.05.035 - “Qiliqiangxin alleviates Ang II-induced CMECs apoptosis by downregulating autophagy via the ErbB2-AKT-FoxO3a axis” by Fuhai Li, Jingfeng Wang, Yu Song, Dongli Shen, Yongchao Zhao, Chaofu Li, Mingqiang Fu, Yanyan Wang, Baozheng Qi, Xueting Han, Aijun Sun, Jingmin Zhou and Junbo Ge, 27 February 2021, Life Sciences.
DOI: 10.1016/j.lfs.2021.119239 - “Traditional Chinese medicine qiliqiangxin attenuates phenylephrine-induced cardiac hypertrophy via upregulating PPARγ and PGC-1α” by Rong-Rong Gao, Xiao-Dong Wu, Hui-Min Jiang, Yu-Jiao Zhu, Yan-Li Zhou, Hai-Feng Zhang, Wen-Ming Yao, Yong-Qin Li and Xin-Li Li, 26 April 2018, Annals of Translational Medicine.
DOI: 10.21037/atm.2018.04.14 - “Qiliqiangxin improves cardiac function and attenuates cardiac remodelling in doxorubicin-induced heart failure rats” by Xutao Sun, Guozhen Chen, Ying Xie, Deyou Jiang, Jieru Han, Fei Chen andYunjia Song, 19 May 2023, Pharmaceutical Biology.
DOI: 10.1080/13880209.2020.1761403 - “Qiliqiangxin Modulates the Gut Microbiota and NLRP3 Inflammasome to Protect Against Ventricular Remodeling in Heart Failure” by Yingdong Lu, Mi Xiang, Laiyun Xin, Yang Zhang, Yuling Wang, Zihuan Shen, Li Li and Xiangning Cui, 13 April 2022, Frontiers in Pharmacology.
DOI: 10.3389/fphar.2022.905424
Funding: National Key Technologies R&D Program (CN, Project No. 2017YFC1700500, 2017YFC1700505); Key Program of National Natural Science Foundation (CN, Project No. 81730106); General Program of National Natural Science Foundation (CN, 81970339, 82270394, 82200425). Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijazhuang, People’s Republic of China) provided part of the funding and the study drug for this research. All funding sources were not involved in the design of the study and collection, enrolment, statistical analysis, interpretation of data and in writing the manuscript.
Disclosures: Prof. Xinli Li reports receiving grant support (all grant support listed paid to the First Affiliated Hospital with Nanjing Medical University) from Novartis and China Heart Failure Center, receiving lecture fees and consulting fees from AstraZeneca, Bayer, Novartis, Roche, and Yiling.

New research shows qiliqiangxin, a traditional Chinese medicine, effectively reduces hospitalizations and cardiovascular deaths in heart failure patients with reduced ejection fraction (HFrEF). Credit: SciTechDaily.com
Qiliqiangxin, a traditional Chinese herb blend, effectively reduces heart failure hospitalizations and deaths, proving safe and beneficial in a large-scale clinical trial.
The traditional Chinese medicine qiliqiangxin reduces hospitalization for heart failure and cardiovascular death in patients with heart failure and a reduced ejection fraction (HFrEF), according to late-breaking research presented in a Hot Line session at ESC Congress 2023.[1]
Qiliqiangxin is a traditional Chinese medicine extract obtained from 11 types of herbs (Table 1).[2] In a pilot study, qiliqiangxin reduced N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels and improved heart failure symptoms in patients with HFrEF when added to established heart failure treatment.3 Preclinical studies have also indicated that qiliqiangxin has beneficial effects on attenuating myocardial fibrosis and cardiac remodeling.[4-7]

Table 1: Ingredients of qiliqiangxin. Credit: European Society of Cardiology
The QUEST Trial: A Comprehensive Evaluation
The QUEST trial evaluated the clinical efficacy and safety of qiliqiangxin on major heart failure outcomes in HFrEF patients. The trial was conducted at 133 hospitals in mainland China and Hong Kong SAR of China.
The trial enrolled adult HFrEF patients with a left ventricular ejection fraction of 40% or below and NT-proBNP of 450 pg/ml or higher who had been on a stable standardized baseline treatment regimen for at least two weeks prior to enrolment. Patients were randomized in a 1:1 fashion to receive qiliqiangxin (four capsules, three times daily) or placebo on top of standard medications for chronic heart failure. The primary endpoint was a composite of rehospitalization for worsening heart failure or cardiovascular death.
A total of 3,110 patients were included in the analysis, with 1,555 randomized to qiliqiangxin and 1,555 randomized to placebo. The average age was 62 years and 72.1% were men. At baseline, the mean left ventricular ejection fraction was 32%, and the median NT-proBNP was 1730.80 pg/ml.
Significant Reduction in Heart Failure Outcomes
During a median follow-up of 18.3 months, the primary endpoint occurred in 389 patients (25.02%) in the qiliqiangxin group and in 467 patients (30.03%) in the placebo group (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.68 to 0.90; p<0.001). This effect was related to both lower risks of rehospitalization for worsening heart failure (HR, 0.76; 95% CI, 0.64 to 0.90; p=0.002) and cardiovascular death (HR, 0.83; 95% CI, 0.68 to 0.996; p=0.045) in the qiliqiangxin group. The effect of qiliqiangxin on the primary outcome was generally consistent across prespecified subgroups including in the subgroups defined according to age and NT-proBNP level, and in patients with or without angiotensin receptor/neprilysin inhibitors (ARNIs).
Secondary Findings and Safety Analysis
In terms of secondary endpoints, the decrease in serum NT-proBNP between baseline and three-month follow-up was greater in the qiliqiangxin group (-444.00 [interquartile range -1401.00 to 85.00]) than in the placebo group (-363.00 [interquartile range -1280.00 to 183.00]) (p=0.047), which was consistent with the previous pilot study.3[]
Analysis of safety endpoints demonstrated no significant difference in all-cause mortality, which occurred in 221 patients (14.21%) in the qiliqiangxin group and 262 patients (16.85%) in the placebo group (HR, 0.84; 95% CI, 0.70 to 1.01; p=0.058). Qiliqiangxin capsules were well-tolerated, with no major differences between the two groups in adverse events including gastrointestinal symptoms, worsening renal function, and increased liver enzymes.
Expert Opinion and Conclusion
Principal investigator Professor Xinli Li of the First Affiliated Hospital of Nanjing Medical University, Nanjing, China said: “To our knowledge, this was the first randomized, double-blind controlled trial of a traditional Chinese medicine for the treatment of chronic heart failure. Our findings demonstrate meaningful clinical benefit with qiliqiangxin in patients with HFrEF, which support the use of qiliqiangxin as an adjunct therapy for treating heart failure.”
References
- QUEST was discussed during Hot Line 2 in room Amsterdam.
- Reference: “Study protocol for a randomized controlled trial: Qiliqiangxin in heart failUre: assESsment of reduction in morTality (QUEST)” by Wenming Yao, Iokfai Cheang, Shengen Liao, Yanli Zhou, Fang Zhou, Dongjie Xu, Zhenhua Jia, Liping Chang, Haifeng Zhang and Xinli Li, 5 February 2020, BMC Complementary Medicine and Therapies.
DOI: 10.1186/s12906-020-2821-0 - “A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study of the Effects of Qili Qiangxin Capsules in Patients With Chronic Heart Failure” by Xinli Li, Jian Zhang, Jun Huang, Aiqun Ma, Jiefu Yang, Weimin Li, Zonggui Wu, Chen Yao, Yuhui Zhang, Wenming Yao, Boli Zhang and Runlin Gao, 7 June 2013, Journal of the American College of Cardiology.
DOI: 10.1016/j.jacc.2013.05.035 - “Qiliqiangxin alleviates Ang II-induced CMECs apoptosis by downregulating autophagy via the ErbB2-AKT-FoxO3a axis” by Fuhai Li, Jingfeng Wang, Yu Song, Dongli Shen, Yongchao Zhao, Chaofu Li, Mingqiang Fu, Yanyan Wang, Baozheng Qi, Xueting Han, Aijun Sun, Jingmin Zhou and Junbo Ge, 27 February 2021, Life Sciences.
DOI: 10.1016/j.lfs.2021.119239 - “Traditional Chinese medicine qiliqiangxin attenuates phenylephrine-induced cardiac hypertrophy via upregulating PPARγ and PGC-1α” by Rong-Rong Gao, Xiao-Dong Wu, Hui-Min Jiang, Yu-Jiao Zhu, Yan-Li Zhou, Hai-Feng Zhang, Wen-Ming Yao, Yong-Qin Li and Xin-Li Li, 26 April 2018, Annals of Translational Medicine.
DOI: 10.21037/atm.2018.04.14 - “Qiliqiangxin improves cardiac function and attenuates cardiac remodelling in doxorubicin-induced heart failure rats” by Xutao Sun, Guozhen Chen, Ying Xie, Deyou Jiang, Jieru Han, Fei Chen andYunjia Song, 19 May 2023, Pharmaceutical Biology.
DOI: 10.1080/13880209.2020.1761403 - “Qiliqiangxin Modulates the Gut Microbiota and NLRP3 Inflammasome to Protect Against Ventricular Remodeling in Heart Failure” by Yingdong Lu, Mi Xiang, Laiyun Xin, Yang Zhang, Yuling Wang, Zihuan Shen, Li Li and Xiangning Cui, 13 April 2022, Frontiers in Pharmacology.
DOI: 10.3389/fphar.2022.905424
Funding: National Key Technologies R&D Program (CN, Project No. 2017YFC1700500, 2017YFC1700505); Key Program of National Natural Science Foundation (CN, Project No. 81730106); General Program of National Natural Science Foundation (CN, 81970339, 82270394, 82200425). Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijazhuang, People’s Republic of China) provided part of the funding and the study drug for this research. All funding sources were not involved in the design of the study and collection, enrolment, statistical analysis, interpretation of data and in writing the manuscript.
Disclosures: Prof. Xinli Li reports receiving grant support (all grant support listed paid to the First Affiliated Hospital with Nanjing Medical University) from Novartis and China Heart Failure Center, receiving lecture fees and consulting fees from AstraZeneca, Bayer, Novartis, Roche, and Yiling.
